Radioactive Decay of Substances : IIT JEE Radioactive Decay of Substances

Radioactive decay is an important topic of the IIT JEE syllabus. Various topics covered under radioactivity like Alpha, Beta and Gamma rays assume great importance because questions are often picked up from these topics in JEE. It is important to master the topic to remain competitive in Practical questions framed on this topic are simple as well as interesting. The JEE does not ask very tricky questions from these heads. Several topics are included under radioactivity but the prime ones are listed below:
- Radioactivity
- Properties of Alpha, Beta and Gamma rays
- Half Life
- Mean Life
- Practical Questions
All these topics have been discussed in detail in the coming sections. We shall give only a brief outline of each of these heads here
Radioactivity : Radioactive Decay of Substances
Radioactivity is basically the process of decomposition of nuclei. The unstable atomic nuclei spontaneously decompose to form nuclei with a higher stability. This process which occurs or the attainment of stability is called radioactivity. The process leads to the emission of energy and particles which are called radiation. There can be two kinds of radioactivity- natural or induced radioactivity.

Natural Radioactivity: When unstable nuclei decompose in nature, it is termed as natural radioactivity.
Induced Radioactivity: As the name suggests, when unstable nuclei are prepared in the laboratory, the decomposition is termed as the induced radioactivity.
Law of Radioactive Decay: The law of radioactive decay envisages how the reduction in number of non-decayed nuclei of a given radioactive substance occurs in due course of time. In other words it may also be stated as the number of atoms disintegrated per second at any instant is directly proportional to the number of radioactive atoms actually present in the sample at that instant.

If N0 be the total number of atoms at t = 0, N be the total number of atoms left in the sample at time t then dN/dt will be the rate of disintegration.
Radioactive Half-Life:
The half-life for a given radioisotope denotes the time for half the radioactive nuclei in any sample to undergo radioactive decay. After two half-lives, the leftover portion will be one fourth of the original sample. Similarly, after three half-lives, one eighth of the original sample and so on. In other words, radioactivity may also be defined as the interval of time required for one-half of the atomic nuclei of a radioactive sample to decay or the time interval required for the number of disintegrations per second of a radioactive material to decrease by one-half.
This decaying of the radioactive substance is based entirely on probability. The half-life is independent of the physical state, temperature, pressure or chemical compounds in which the nucleus finds itself. Hence no external factors can have an influence on the half-life. In fact, the only thing that can have an impact on the high-life of a radioactive particle is the direct nuclear interaction with an external particle. For example, a high energy collision in an accelerator might have an impact.
Average Life or Mean Life
In a given sample, all the atoms do not undergo disintegration together. Some atoms may disintegrate in the very beginning for which the lifetime is zero. Hence, in case of atoms which disintegrate in between, their lifetime may vary from zero to infinity. The total lifetime of all the atoms of the elements divided by the total number of atoms present initially in the sample of the element is called the Mean life.
Alpha, Beta and Gamma Rays: Radioactive Decay of Substances
The rays emitted during radioactivity of a substance are called alpha rays, beta rays and gamma rays. We discuss the properties of all three one by one.
Alpha Radiation:
Alpha rays are the positively charged radiation. These rays are more massive than the beta rays and they contain a stream of positively charged particles, called alpha particles, which have the atomic mass of 4 and a positive charge of 2 (a helium nucleus). The emission of an alpha particle from the nucleus decreases the mass number of the nucleus by 4 and the atomic number reduces by 2. As in the reaction given below, the helium nucleus is the alpha particle.

The alpha radiations are made up of two neutrons and two protons which are attached to the nucleus of a helium atom. These particles have an intense ionization power which means that the moment they come into contact with atoms of a living tissue they have the ability of causing mutations and at times such reactions may even lead to cancer. Despite of their high ionization power these rays are not very harmful. In fact, out of the three rays, these are the least dangerous as long as they are not inhaled. Alpha rays cannot penetrate through the skin and can even be stopped by a few centimeters of air.
Beta Radiation:
Beta radiations consist of a stream of electrons termed as beta particles. On the emission of a beta particle, a neutron in the nucleus gets converted to a proton and hence the mass number remains unchanged but the atomic number increases by one unit. Consider the example given below in which the electron is the beta particle:

23490 → 0-1e + 23491Pa
Beta rays are made up of high energy electrons. They are less ionizing than the alpha rays but are more harmful as they can penetrate through the skin .they can be stopped with an aluminum sheet.
Gamma Radiation
Gamma radiations are extremely high energy photons with a very short wavelength (0.0005 to 0.1 nm). Alpha and beta emission are often accompanied by gamma emission, as an excited nucleus drops to a lower and more stable energy state. Gamma rays are electromagnetic waves of high frequency with no mass and no charge. They have the least ionizing power but are most dangerous. Gamma rays have the highest penetrating power and can only be stopped by a few centimeters of lead or few meters of concrete. In certain cases they may even pass through them.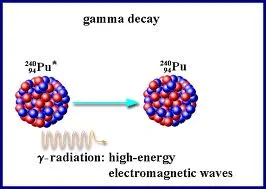
Radioactivity and the three types of rays constitute an interesting and vital topic of the IIT JEE. Once the concepts and the difference between the types of rays are clear, mastering numerical is not a big deal.
